FOSTERING
SCIENTIFIC TEAM-WORK:
TRAINING
UNDERGRADUATES TO BE
EFFECTIVE
COLLABORATORS.

The Department of
Chemistry at Hobart & William Smith Colleges is involved in developing and
implementing a new laboratory curriculum which is modeled after the interdisciplinary,
team-focused methods of investigation that occur in post- baccalaureate
professional and industrial settings.
The cooperative learning projects interleave synthetic and spectroscopic
methodologies within the interdisciplinary umbrella of computer-aided molecular
modeling. This project has been
generously supported by the Camille and Henry Dreyfus Foundation and the
Patchett Foundation.
Description
and Significance of the Project
The Department of
Chemistry intends to develop a new model for scientific instruction. The complex nature of science and technology
requires that scientists are trained not only rigorously within a discipline,
but also with an understanding of, and appreciation for, other disciplines. The model we propose incorporates
cooperative, interactive laboratory projects into the curriculum, not only
within courses, but between courses as well.
We intend to introduce this discovery-based, “working-in-groups” method
for learning chemistry at all levels of our curriculum. Previous studies have shown that cooperative
learning and the development of improved communication skills were directly
correlated with increased performance and retention among chemistry students.[1] We will be teaching chemistry the way
chemistry is done, choosing from among a powerful and diverse set of tools with
which to attack real world problems.
These projects will help students to develop the skills necessary to
work together effectively, and to utilize their distributed expertise
synergistically. Interactive projects
will attract and cultivate student interest in the basic sciences and an
emphasis on computer-aided visualization will increase the diversity and
numbers of students electing chemistry as a major. We have chosen molecular modeling as the basis of the inter-class
link because it is a multidisciplinary area of science that transcends the
traditional boundaries that separate biology, chemistry, physics and computer
science. There have been a number of
reports in the chemical education literature emphasizing the importance of
computational chemistry in the undergraduate curriculum.[2],[3],[4],[5]
The Department of
Chemistry has recently committed to a major restructuring of the chemistry curriculum
to encourage cross-disciplinary connections in ways that are seamless,
rigorous, and encourage the retention of material and enjoyment of the
subject. As a logical first step in our
curriculum reform, we are developing laboratory projects not only within and
between chemistry courses, but between courses in different science departments
as well. The inter-class laboratory projects will be incorporated at
all levels of our curriculum and will enable students to i.) learn to work and
communicate effectively together, ii.)
discover, first hand, the excitement that comes from solving real world
scientific problems, and iii.) be
systematically exposed to modern, technologically sophisticated scientific
techniques. Our goal is to have every
one of the 250+ students who annually take chemistry courses at Hobart and
William Smith Colleges participate in a project-oriented, collaborative
approach to learning. The model we have
developed is applicable across the chemistry curriculum but is dependent upon
the availability of molecular modeling laboratory equipped with
graphics-capable computer workstations.
We are currently developing new laboratory based projects and have
successfully incorporated the following examples:
Collaborative Laboratory
Connecting Chem. 240, Organic Chemistry
II and Chem. 320, Physical Chemistry I: As one example of
the types of collaborative laboratory exercises that we envision, the
conformational behavior of a series of Mosher amides will be investigated using
NMR spectroscopy and molecular modeling.
Derivatization of optically active amines as Mosher amides is a
convenient and reliable way to obtain quantitative information about the
absolute stereochemistry of the underivatized amine. The nonequivalence of the NMR spectra of diastereomeric Mosher
amides can be used to determine the enantiomeric purity of the amines. The Mosher argument assumes that the most
stable conformation of the (S)–amide is as shown in 1. This argument can be
confirmed and visualized using molecular modeling of the diastereomeric Mosher
amides.
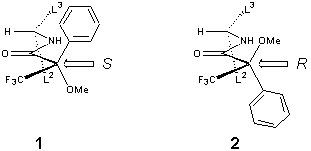
In the (S )–amide(1) protons in the L3 portion of the molecule are shifted
upfield while those in the L2 portion of the molecule appear further
downfield. Organic chemistry students
will be given optically pure amines and will work in teams to derivatize the
amines with both enantiomers of Mosher acid chloride. Simultaneously, the physical chemistry students will utilize
molecular modeling methods to identify the lowest energy conformation of each
diastereomer. The organic and physical
chemistry students will then work together in sub-disciplinary, inter-class
teams, describing their individual contributions to the overall project. Each sub-disciplinary team will then record
the NMR spectra of the diastereomeric Mosher amides and deduce the absolute
configuration of the starting amine.
This particular
laboratory was our first experiment to be implemented. In the semester prior to the introduction of
the laboratory, the project director worked with our organic chemist and an
independent study student to develop the laboratory materials and techniques.
Then, twelve physical chemistry students worked together with twelve
organic chemistry students (a subset of the total number of organic students
for the year due to a lack of workstation resources).
The results from this pilot were very encouraging, as judged by i.)
the results and comments from our traditional, formal student course evaluations,
ii.) the results from an evaluation form designed to assess an understanding
and retention of the material presented, and iii) informal conversations with
students and observations of student enthusiasm for the format.

Collaborative Laboratory
Connecting Chem. 322, Physical
Chemistry II and Chem. 448, Biochemistry I: Another example of the types of
collaborative laboratory exercises that we plan to develop involves the use of
molecular modeling and nmr spectroscopy to investigate the solution structure
for Angiotensin I. This laboratory will
likely extend over a number of weeks in the term, allowing students to develop
strong working relationships.
Biochemistry and physical chemistry students will work together to build
an energy minimized solution-phase model of Angiotensin by investigating its
conformational flexibility using the Amber force field combined with a
continuum approach to solvation. In the
second week, students work together to collect and assign a 1D proton and C13
spectrum of simple amino acids and small peptides in D2O. Using the smaller peptides, students will
generate a library of spectra to assist in making assignments on the more
complex Angiotensin. In week three,
students will take the 1D proton spectrum of angiotensin and use this
information to verify the amino acid composition and sequence. Next, in week four, the student will learn
about and collect 2D COSY and NOESY spectra for Angiotensin. Students will use this data to identify
dihedral angle and interatomic distance constraints, which can be used to
further refine the computer model for the 3D structure. In the last week of the laboratory, students
will learn together about molecular dynamics and use these methods to
investigate the local solution-phase behavior of Angiotensin, subject to
experimental constraints. This laboratory
will encourage students to work together effectively and consistently over an
extended period of time on a project-oriented problem, to connect information
learned from different courses and to experience, first hand, the
inter-relationship between theory and experiment, molecular models and observed
behavior.
Collaborative Laboratory
Connecting Chem. 322, Physical
Chemistry II and Chem. 436, Inorganic Chemistry: This laboratory exercise utilizes
inorganic synthesis, electronic absorption spectroscopy and molecular modeling
to prepare and characterize cobalt (III) ammine complexes and to investigate
the effect different ligands have on the conductivity and electronic transition
spectra. Inorganic students will
synthesize a series of cobalt complexes:
[ Co (NH3)5 X ] +3-l
Yy
where X-l represents a series of different
ligands and Yy represents the counter ion(s).
The physical chemistry students will perform a series of quantum
calculations on small cobalt complexes to develop a suitable molecular mechanics
force field for these systems.
Conformational searching will be performed on each complex synthesized,
locating all low energy structures and evaluating the corresponding energies. The inorganic and physical chemistry
students will then work together in sub-disciplinary, inter-class teams,
describing their individual contributions to the overall project. Sub-disciplinary teams will coordinate the
collection of electronic absorption spectra for each complex and i.) assign ligand field transitions, ii)
evaluate energy differences between reactants, products and possible
intermediates and iii.) compare reaction conditions with energetic information
to determine the kinetic/thermodynamic product, for the various
ligand-substituted complexes.
Collaborative Laboratory
Connecting Chem. 110, General Chemistry
and Chem. 240, Organic Chemistry:
One of the experiments utilized in the organic/introductory chemistry
collaboration is a simple, fun experiment that relates the "fruity"
odors of acetate esters to their molecular structure. The students will explore first hand the issues and problems
confronted by flavor and fragrance chemists.
Various esters can be prepared easily and inexpensively by the organic
students via Fisher esterification. The
general chemistry students will use molecular modeling software to build and
energy minimize the molecules that are synthesized. The organic and general chemistry students then work together to
calculate and tabulate a number of molecular descriptors such as dipole moments
and molecular volumes and surface areas.
This information is used to develop a relationship between molecular
structure and function, in this case, odor.
By enabling our first and second year chemistry students to use
"technologically sophisticated" workstations to visualize molecular
phenomenon, we hope to build their confidence, interest and desire to continue
in science.
We intend to build
at least one collaborative laboratory bridge between each of our traditional
courses in chemistry. We plan to
stagger the collaborations utilized every other year, so that, for instance, a
chemistry/biochemistry major will have numerous opportunities to participate in
collaborations without having to repeat a particular project. Table 1 provides a sampling of other
inter-class projects that we envision.
Table 1.
Inter-Class Cooperative Learning Projects.
(N is the average class enrollment per term, laboratory sizes are never
larger than 20)
|
Class |
N |
Class |
N |
Project |
|
Physical Chemistry
I. |
15 |
Chem. 110 General
Chemistry I |
70 |
Conformational
entropy/enthalpy |
|
Chem. 436 Inorganic
Chemistry |
10 |
Chem. 241 Organic
Chemistry |
45 |
conf.
flexibility about a metal center |
|
Organic
Chemistry II |
10 |
Chem. 322 Physical
Chemistry II |
Experimental/theoretical
study of Diels-Alder rxns |
|
|
Organic
Chemistry |
10 |
Chem. 320 Physical
Chemistry |
45 |
Experimental/theoretical
study of Hammond postulate |
|
Chem. 348 BioChemistry I |
15 |
Chem. 322 Physical
Chemistry II |
10 |
protein
inhibition, enzyme kinetics |
|
Chem241 Organic
Chemistry II |
45 |
Chem. 449 BioChemistry
II |
10 |
drug design |
|
Chem. 449 BioChemistry
II |
10 |
Chem. 322 Physical
Chemistry II |
10 |
DNA
intercalation |
Information about Hobart and
William Smith Colleges
 Hobart College for men, founded
in 1822, and William Smith College for women, founded in 1908, comprise a
coordinate, residential liberal arts and science institution. The Colleges share a common curriculum and faculty while offering
students the benefits of both a single-sex and coeducational environment by
maintaining separate residential, athletic and student government programs.
This unique environment enables men and women to enjoy a range of leadership
and discovery opportunities.
Hobart College for men, founded
in 1822, and William Smith College for women, founded in 1908, comprise a
coordinate, residential liberal arts and science institution. The Colleges share a common curriculum and faculty while offering
students the benefits of both a single-sex and coeducational environment by
maintaining separate residential, athletic and student government programs.
This unique environment enables men and women to enjoy a range of leadership
and discovery opportunities.
The educational
objective of the Colleges is to provide an academically rigorous liberal arts
and science education with a strong emphasis on interdisciplinary work. There are 134 faculty with 98% of the
faculty holding the Ph.D. or equivalently terminal degree. Women comprise 40% of the faculty, and women
scientists total 35% of the science faculty.
There is a heavy emphasis on teaching, and research and publishing with
undergraduates is expected. Admission
to the Colleges is rated as highly selective.
The combined enrollment at Hobart and William Smith Colleges for the Fall of 1997 was 1780 undergraduates
with a nearly equal distribution among men and women. The faculty to student
ratio is 13:1.
Hobart and William
Smith Colleges has developed a highly effected program for recruitment and
retention of minority students. In the
fall of 1997, 18% of the first year
students were minority U.S. citizens or foreign nationals of minority
background. Academic support services
for minority students are coordinated by the associate dean for intercultural
affairs who also administers the Colleges Higher Education Opportunity Program
(HEOP). This program which is funded by the State of New York, assists students
who are economically and educationally disadvantaged. Hobart and William Smith has achieved the highest retention and
graduation rate of all HEOP programs in the state, with an average of 77% of
all participants completing their degrees within six years.
Graduation requirements
reflect the Colleges commitment to academically rigorous liberal arts and
science education in an interdisciplinary environment. All students must complete two majors (or a
major and a minor), one of which must be disciplinary while the other must be
interdisciplinary in nature. The
William R. Kenan Jr. Charitable Trust recognized the Colleges for its
excellence in undergraduate teaching with the award of a $2 million challenge
grant to endow a William R. Kenan Jr. Professorship and encourage the formation
of a $3 million endowed faculty development fund. The Christian A. Johnson Endeavor Foundation has also recognized
the College’s innovative leadership in curriculum and pedagogy development with
three awards totaling nearly two million dollars for ongoing support and
endowment.
Chemistry
at Hobart & William Smith Colleges
The chemistry
department at Hobart and William Smith Colleges offers a range of intellectual,
collegial and mentoring opportunities for students and faculty alike. We
currently have five Ph.D. faculty occupying tenured or tenurable positions,
three visiting professor positions, a senior research scientist and one full
time laboratory technician. The youth
and enthusiasm of our new faculty, combined with the strength and stability of
our mid-career full professors will enable HWS chemistry to make a quantum leap
forward in teaching and research excellence.

One measure of the
quality of the chemistry faculty is the number of chemistry and biochemistry
graduates going on to advanced study or employment in the chemical
sciences. Our survey of graduates in
the past ten years indicate that 24 of 62 majors in chemistry, or 38%, have
entered graduate programs in the chemical sciences, 25% have chosen careers in
the chemical industry and 26% have pursued medical or other professional
programs. These statistics indicate
that students attracted to the sciences at Hobart and William Smith are well
served by their training and are strongly motivated to continue in
science. Another measure of the
robustness of the department is the rapid growth of the biochemistry
major. This program has grown from 3
majors since it’s inception in1994 to 20 in 1998. This growth complements the department’s historically steady,
10-12 chemistry majors declared each year.
The chemistry
department prides itself in offering to every one of its majors the opportunity
to do research, one-on-one, with a faculty member. Students can do research during the summer with stipend and
housing support from the Provost’s Office or the departmental Merck/AAAS
Undergraduate Science Research Grant.
The level of student research activity in the department is significant
and 80% of our majors participate in research even though it is not a
requirement for the major. Students
often present the results of their research at scientific meetings. At the last national American Chemical
Society meeting in Boston, 16 Hobart and William Smith students presented their
work.
The Colleges are
recognized for their commitment to encouraging women to pursue careers in
science. This reputation was recently
confirmed by a national survey of undergraduate science programs at selective
liberal arts colleges, which ranked the Colleges among the leading
baccalaureate sources of natural science doctorates earned by women. In the past five years, 65% of our chemistry
majors have been women, and more than half of these women have gone on to
pursue advanced study in the chemical sciences.
Institutional
Support of the Project

The Colleges have
committed to providing the space and resources necessary to renovate an
existing wet laboratory to a molecular modeling facility. This includes the removal of fume hoods,
benchtops, false walls and plumbing, and the installation of static-free
carpeting. The Colleges have also
committed the funds necessary to equip the room with the appropriate computer
furniture, bookcases and ample electrical source.
Resources
Available for the Project
The chemistry
department is fortunate to have a wide variety of powerful spectrometers with
which to develop new laboratory experiments. The use of modern spectroscopy in
lower-level collaborative projects will allow students, science and non-science
alike, to gain familiarity and appreciation of techniques and skills they might
not otherwise obtain. In the past five
years, the department was the recipient of three NSF-ILI grants for: a 300 MHz nuclear magnetic resonance
spectrometer (#USE-9250347), an inductively coupled plasma spectrometer (#USE-9250655)
and a GC-MS spectrometer equipped with an autosampler (#DUE-9751652/1997
Dreyfus Special Grant Award). Also
available is a Perkin Elmer DSC/TGA, a Perkin Elmer 559A UV/Vis, a Mattson
Galaxy 2020 FT-IR, a Perkin Elmer AA-372 Atomic Absorption, a Perkin Elmer
650-10S Fluorescence, a Beckman HPLC/IBM PC and Leica Quantimet Image Analysis
system. Computational chemistry
software is readily available as the department currently holds licenses to run
MacroModel, Jaguar, Delphi, GAMESS and SynLib on an unlimited number of
computers. The PI has five Silicon
Graphics O2 workstations, and these will be available for use in the
collaborative projects, however they are used heavily in undergraduate research
projects, and the computational cycles available will be limited.
Implementation and Equipment Maintenance
The project
director (C. Parish) assumes responsibility for system administration of the
molecular modeling laboratory, including networking, routine maintenance and
trouble shooting. For more serious
repairs the chemistry department has committed the necessary support. We plan a for a molecular modeling
laboratory capable of serving classes of 24-48 students, with teams of 2–4
students per workstation. Our largest
laboratory section in chemistry is limited to 20 students, so the modeling
laboratory will easily accommodate even collaboration between two of our
largest sections.
Evaluation and Dissemination
The
Hobart and William Smith chemistry department is an ideal setting for
experimenting with cooperative, inter-class learning strategies owing to its
flexibility and commitment to scientific rigor. In order to assess the impact of this change in our curriculum,
we plan to carefully evaluate i.) the results and comments from our
traditional, formal student course evaluations, ii.) the results from an
evaluation form designed to assess an understanding of the material, comparing
differences in understanding and content mastery between students who had been
exposed to the material via a collaborative lab versus those who were exposed
in a more traditional, non-collaborative format, and iii) the results of
post-course, exit and post-graduate interviews with students.
Our
aspiration is for the results of these experiments and innovations to be useful
in the national reform of science curricula.
We intend to report the results of these inter-class projects at
national chemistry meetings.
Preliminary work on our use of the Mosher amide cooperative laboratory
as a pilot has already been presented at the 215th National American Chemical
Society meeting in Dallas. This
presentation initiated a number of requests for laboratory materials from
departments at other institutions, which we obliged. Articles describing our
findings will be submitted to the Journal of Chemical Education and published
on our departmental web page for widespread dissemination.
Encouraged
by the enthusiastic response at the Dallas ACS meeting, we intend to volunteer
in the Division of Chemical Education to organize a symposium focusing on
collaborative learning at an upcoming national ACS meeting. We would also contact pharmaceutical and
chemical companies for support of this symposium and encourage the
establishment of industrial grants and scholarships for the implementation of a
collaborative approach to laboratory work at other institutions. We plan to use the majordomo facilities at
HWS to establish a USENET news/discussion group which anyone on the internet
interested in collaborative learning could read and contribute to. The symposium, major developments and the
establishment of the discussion group would be disseminated via postings to
existing electronic list, such as the Council on Undergraduate Research list
(cur-l) and the Computational Chemistry list (CCL). Finally, we would contact other institutions with large scale
efforts underway at curriculum reform, and explore ways of dovetailing our
efforts with theirs.